Copper Refining Rectifier: The Key to High-Purity Electrolytic Copper Production
Overview of Copper Refining
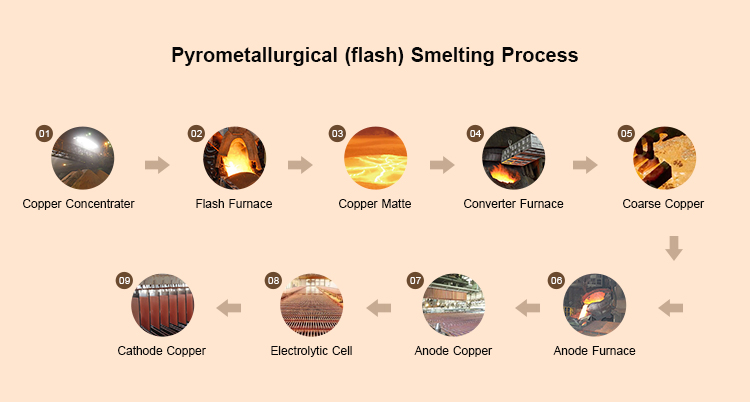
Copper refining refers to the process of purifying crude copper (copper with high impurities) through physical or chemical methods to obtain high-purity copper. Coarse copper is usually extracted from copper ore by pyrometallurgy and contains various impurities such as gold, silver, iron, nickel, sulfur, etc.

The Purpose of Electrolytic Refining
The main purpose of copper refining is to remove these impurities, improve the purity and performance of copper, and make it suitable for industrial applications.

Copper refining is usually divided into two stages
Fire Refining
Melt crude copper and add oxidants (such as air or oxygen) to oxidize impurities (such as iron, sulfur, etc.). Impurities form oxides, float on the surface of the copper liquid, form slag, and are removed. The remaining copper with a purity of about 98-99% is called anode copper and is used for subsequent electrolytic refining.
Electrolytic Copper
Make the anode copper obtained by pyrometallurgical refining into an anode plate and place it in an electrolytic cell. The electrolytic cell contains electrolytes of copper sulfate (CuSOx) and sulfuric acid (H ₂ SOx). After being powered on, the anode copper dissolves, and copper ions (Cu ² ⁺ ) migrate to the cathode and deposit as high-purity copper on the cathode. Electrolytic copper is the final product of copper refining. Electrolytic refining is the final step in copper refining, determining the final purity of copper.
Copper Deposition is the Core Step of Copper Refining
In the process of electrolytic refining, copper deposition is a key purification step, with anodic copper dissolution and copper ions entering the electrolyte. Copper ions are reduced to high-purity copper at the cathode and deposited, forming electrolytic copper. By controlling deposition conditions such as current density and electrolyte composition, impurities can be efficiently removed to obtain high-purity copper.
Copper Deposition for By-product Recovery in Copper Refining
The anode mud generated during the electrolytic refining process contains precious metals (such as gold and silver) and scattered metals (such as selenium and tellurium). These valuable metals can be recovered from anode sludge through chemical deposition or electrochemical deposition.
The Improvement of Copper Deposition Technology Promotes the Development of Copper Refining
The advancement of copper deposition technology, such as efficient electrolytic cell design and additive optimization, has improved the efficiency and product quality of copper refining. For example, by improving sedimentation conditions, energy consumption can be reduced and copper purity can be increased.

Production Process Flow
Copper electrolytic refining is the process of casting copper refined by pyrometallurgical methods into anode plates, using pure copper sheets as cathode plates, and alternately placing them into electrolytic cells. The electrolyte is composed of an aqueous solution of copper sulfate and sulfuric acid. Under the action of direct current, copper on the anode and base metals with negative potential dissolve into the solution.
In the process of copper electrolysis, precious metals and certain metals (such as selenium and sulfur) do not dissolve, but instead become anode sludge and settle at the bottom of the electrolytic cell.

These anode slurries containing precious metals and rare metals such as selenium and sulfur, as a byproduct of copper electrolysis, need to be treated separately in order to recover elements such as gold, silver, selenium, and sulfur from them.
During the electrolysis process, copper ions in the solution preferentially precipitate on the cathode, while other base metals with negative potentials cannot precipitate on the cathode and remain in the electrolyte. These base metals are removed during regular electrolyte purification. Therefore, the metal copper deposited on the cathode has high purity and is called cathode copper or electrolytic copper, abbreviated as electrolytic copper.


Raw Material Preparation
Coarse copper serves as the anode, while pure copper or stainless steel serves as the cathode.
Crude copper contains impurities and needs to be refined and purified to provide the basic material for subsequent electrolysis. Electrolyte configuration, commonly used copper sulfate and sulfuric acid mixed solution. The concentration and acidity of the electrolyte affect the electrolysis efficiency and copper purity.

Electrolytic Process
Under the action of direct current, the current passes through the electrolytic cell, and the copper ions (Cu ² ⁺ ) at the anode dissolve into the electrolyte. Copper ions obtain electrons at the cathode, reduce to pure copper, and deposit on the cathode, resulting in the precipitation of high-purity copper at the cathode. The current density and electrolysis time determine the copper yield and purity. Cathodic copper treatment, washed and dried to produce the product. Improper handling can result in residual impurities and affect copper performance.

Equipment Composition
Electrode
Anode: Made of coarse copper, copper ions dissolve into the electrolyte from here.
Cathode: Made of pure copper or stainless steel, where pure copper is deposited.
Power Supply System
Provide direct current, rectifiers, transformers, cables, etc. to the production line.
Electrolyte Purification System
Remove impurities from the electrolyte and maintain its purity. There are filters, ion exchange equipment, chemical treatment equipment, etc.
Electrolytic Cell
The core equipment for conducting electrolytic reactions typically uses corrosion-resistant materials such as plastic or rubber lined steel or concrete tanks.
Electrolyte Circulation System
Maintain uniform electrolyte composition and stable temperature. Including
circulation pumps, pipelines, heating or cooling devices.
Automatic Control System
Monitor and regulate the electrolysis process. Sensors PLC, Computer control systems, etc.
Anode Mud Treatment Equipment
Collect and process anode sludge, extract precious metals. There are mainly filters, centrifuges, melting furnaces, etc.
The Role of Rectifiers in Copper Refining
Making high-purity copper from raw ore requires precise electrical control. That’s where rectifiers come in – these power converters are the silent workhorses powering modern copper refineries.
Here's how they fit into the process
First, smelters process raw ore into crude copper. Then converters remove more impurities. But the real transformation happens in electrolytic tanks, where:
• Impure copper anodes (about 99% pure) are suspended in acid baths
• Pure copper gradually builds up on starter cathodes
• This entire electrochemical process depends on direct current
Why Rectifiers Are Indispensable
The power grid supplies alternating current (AC), but copper refining requires steady direct current (DC). Rectifiers solve this critical need by:
• Converting incoming AC into the required DC
• Providing the exact power specifications needed for refining
• Maintaining current stability within tight tolerances – crucial for uniform copper deposition
Advantages of Modern Rectifier Systems
Today’s advanced units outperform older models by delivering:
• Exceptional energy efficiency (over 98% conversion)
• Unwavering current consistency
• Automatic adjustments for varying feedstock quality
Their intelligent control systems make constant micro-adjustments – perhaps increasing voltage slightly during peak demand periods, or decreasing it during off-hours – ensuring every cathode meets exact specifications.
Key Applications in the Industry
Industry Trends Shaping Development
With growing demand for copper in electric vehicles and renewable energy, refineries now require rectifiers that can:
• Support expanded production capacities
• Withstand challenging operating environments
• Integrate with intermittent renewable energy sources
The Critical Importance
Without rectifiers, modern copper refining simply wouldn’t exist. These systems directly determine:
• Whether cathodes achieve 99.99% purity standards
• The plant’s overall energy efficiency
• The ability to process recycled materials
Future Innovations on the Horizon
Next-generation rectifiers will feature:
• Automatic grid disturbance compensation
• Enhanced cooling for extreme climates
• Smart energy management capabilities
As the copper industry moves toward sustainable production, these power conversion systems will continue enabling efficient purification while adapting to new energy challenges.
Related Cases
Copper Refining Rectifier Helps Waste Copper Recycling Companies to Produce Efficiently
The client specializes in recycling scrap copper. They purchased a 13KA8V thyristor rectifier for copper refining and were highly satisfied with its performance. According to their feedback, the rectifier runs reliably, delivering a stable supply of high-quality DC power to ensure the copper refining process proceeds smoothly. Its precise control keeps electrolysis parameters consistently at optimal levels, helping to increase both copper purity and production output. The equipment is also simple to operate, requires minimal maintenance, and contributes to higher production efficiency and better economic returns.
Welcome to visit
Welcome to contact us for further discuss if you are interested in any of our products.








